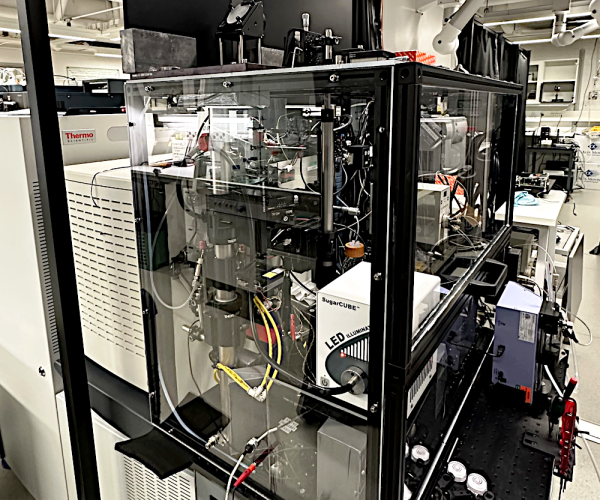
MS imaging platform
Novelty and impact
The novelty and impact of the imaging technology developed is based on the experimental IR-LAAPPI and IR-LAESI ion sources and the combination of their different capabilities, which provide features for the analysis and imaging of biological samples not available on any commercial instrument. The principles of the developed ion sources are described here: Ion sources.
The analysis and imaging of biological samples is matrix-free and label-free, and does not require sample pre-treatment that could introduce new sources of uncertainty or bias. This is important where smaller and smaller changes need to be observed, such as in single-cell analysis.
The metabolic state of the sample is preserved after sample collection as the cold chain is not broken after flash-freezing and cryosectioning. IR-LAAPPI and IR-LAESI can therefore minimise biological bias caused by the fact that many metabolites are unstable or metabolic reactions are ongoing at room temperature.
The ionisation step determines the types of compounds that can be detected by MS, and the platform developed can currently utilise two different types of ionisation, APPI and ESI, while allowing for further additions in the future. This allows the detection of a wide range of polar and non-polar compounds and thus extensive compound coverage, providing a fuller picture of the sample's metabolome.
Imaging platform enables integration of other imaging methods, and in-house built light microscope is already integrated with the platform to enable microscopy-guided analysis of single cells.
Maximum lateral resolution of 10–20 µm for high-resolution imaging, with the ability to vary the sampling spot diameter in the range of 10-80 µm. This allows the sampling spot size to be matched to the cell size.
IR-LAAPPI and IR-LAESI use high-energy infrared laser ablation sampling, sufficient to eject analytes below the sample surface, whereas most MS imaging methods are limited to surface analysis. Fine control of IR laser ablation sampling, synchronised with MS data acquisition, enables depth profiling and 3D imaging. Higher sampling energy is also often required for the analysis of plant tissues, which are often covered by different layers, such as wax, or are more rigid than animal tissues.
All features and capabilities combined, IR-LAAPPI and IR-LAESI compare favourably with commercial MS imaging methods, which often either require extensive sample treatment or cause high fragmentation of biomolecules, preventing their identification. Most methods also operate at room temperature, where many metabolites are unstable and biological reactions are ongoing.
Table 1. Comparison of common mass spectrometry imaging methods for the analysis of biological samples. (IS = ion source, ST = sample treatment, FR = fragmentation, LR = max lateral resolution (order of magnitude), MR = upper mass range limit)
| Method | IS | ST | FR | LR (µm) | MR (Da) | Analytes |
|---|---|---|---|---|---|---|
IR-LAAPPI |
IR laser + APPI | no | slight | 10 | 2000 | polar – nonpolar |
IR-LAESI |
IR laser + ESI | no | minimal | 10 | 10000 | polar – semipolar |
| DESI | charged spray | no | minimal | 10 | 10000 | polar – semipolar |
| UV-MALDI | UV laser + matrix | yes | minimal | 1 | 100000+ | polar – semipolar |
| IR-MALDI | IR laser + matrix | yes | minimal | 10 | 2000 | polar – semipolar |
| SIMS | ion beam | yes | extensive | 0.01 | 2000 | polar – semipolar |
Operating principle
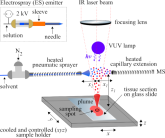
Although the ionisation principles of IR-LAAPPI and IR-LAESI are different, their operating principle is similar
- IR laser beam is focused to a certain spot size
- Laser burst fires pulses of 2.94 µm photons
- IR pulse energy is absorbed by the sample water
- Sample is ejected to the gas phase, intersecting with charged solvent
- Sample compounds are dissolved, ionized and transported into the mass spectrometer
- Ions are separated based on their mass-to-charge (m/z) ratios
- Ions with different m/z values are detected and mass spectra acquired
- The sample is moved and process repeated for imaging
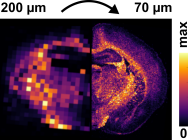
Each spot analysed represents a pixel in MS images and the effect of step on spatial resolution and the ability to spatially resolve histological features is clearly seen in the image below.
The imaging platform developed also has a modular design that allows the use of both methods by simply changing the IR-LAAPPI nebulizer with an electrospray emitter, required for IR-LAESI, as shown in the schematic. For ionization principles and further details, please refer to the Ion source development section below.
Application: Animal tissue
MS imaging is most commonly used to analyse tissue sections of rodent organs that have first been flash-frozen to cryogenic temperatures and then cut into thin slices (i.e., sections) using a cryostat microtome. These methods preserve sample morphology and reduce the rate of ongoing metabolism and sample degradation by keeping the sample frozen. Both IR-LAAPPI and IR-LAESI are therefore well suited for the analysis of such samples as they are able to image frozen tissue sections directly, ensuring minimal sample degradation and metabolic changes between sample collection and the end of the imaging analysis. This also means that IR-LAAPPI and IR-LAESI imaging results accurately represent the collected sample and not its altered forms.
IR-LAAPPI and IR-LAESI can also image samples of varying thickness, typically from 10 to 80 µm, due to the energetic IR laser ablation sampling method. Both methods are also suitable for imaging samples without preparation if they are sufficiently planar, such as the plant leaves shown below. The chosen sample thickness affects the axial resolution of imaging and the number of samples that can be prepared for analysis from a single organ, and can be used to sacrifice resolution in order to increase sensitivity for low concentration compounds.
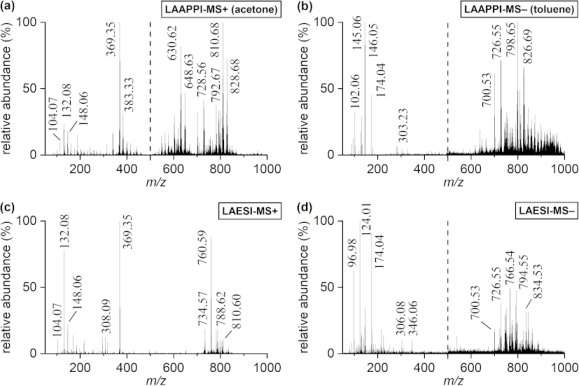
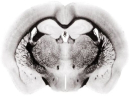
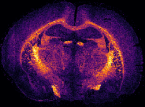
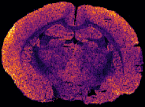
In MS imaging, data is collected from different positions in the sample, resulting in mass spectra as shown above. When the imaging analysis is complete, a single mass peak and its m/z value can be selected from the spectral data, representing a single compound detected in the sample. By plotting the intensity of this mass peak from each analysed position, MS images (heat maps) can be generated to show its relative change and thus how the compound is distributed in the sample area. For example, negative ion IR-LAAPPI MS images of a 20 μm thick slice of whole rat brain tissue measured at 70 μm spatial resolution, consisting of approximately 30,000 pixels, are shown below to illustrate how different lipid species are distributed in the brain.
| Photo | MS image | MS image | MS image |
|---|---|---|---|
 |
 |
 |
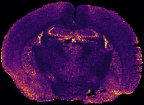 |
| Myelin-stained brain | GalCer(38:1) | PE(34:0) | PE-P(36:4) |
Conventional imaging techniques such as positron emission tomography, autoradiography, and immunohistochemistry, can only image a few radiotracers, radioisotopes, or antibody-labeled molecules at a time, respectively, as presented with the myelin-stained photo above. MS imaging techniques like IR-LAAPPI and IR-LAESI can image hundreds of compounds simultaneously, thereby providing a valuable tool for finding differences between sample types such as malignant and benign tumors.
Application: Plant tissue
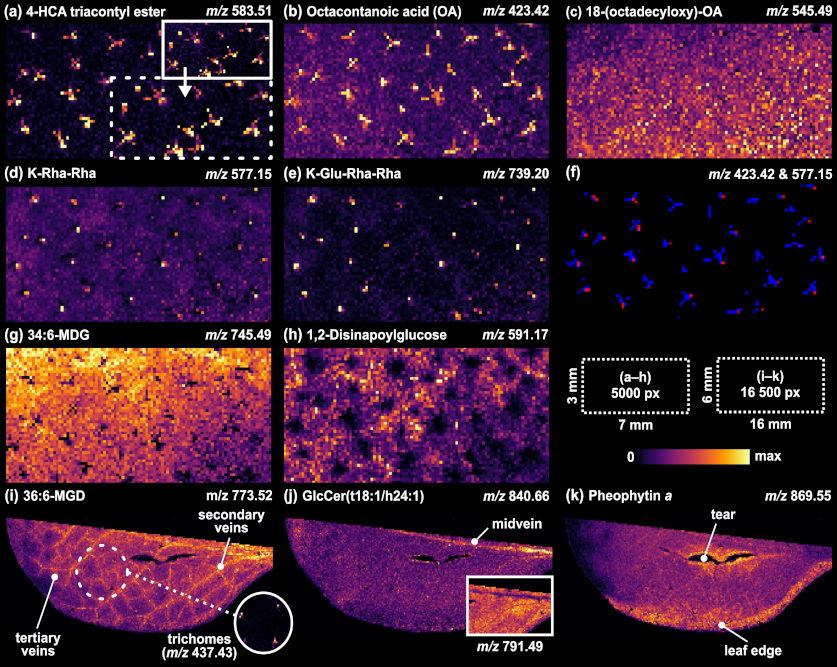
Both IR-LAAPPI and IR-LAESI are cutting edge mass spectrometry imaging techniques for the analysis of plant tissues because of their ability to analyse frozen plant samples with complex structures without sample treatment such as matrix deposition or even preparation such as tissue sectioning. Plant tissues also exhibit rapid metabolic responses immediately after sample collection, but more importantly during conventional imaging analysis at room temperature. Conventional MS imaging techniques are also more or less limited to the analysis of sample surfaces. These are often covered with a layer of wax, especially in leaves.
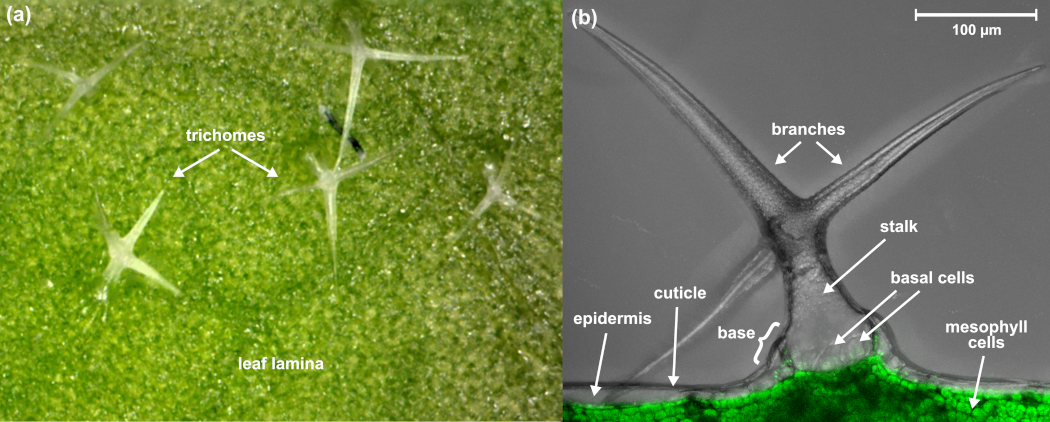
For example, Arabidopsis thaliana (A. thaliana) leaves have a complex, multilayered structure including substructures such as trichomes (plant hair), cuticular and surface waxes, veins, and epidermis, which can be difficult to analyse and spatially resolve from each other with more traditional MS imaging methods, such as MALDI.
IR-LAAPPI MS imaging with 70 μm lateral resolution has been shown to allow the analysis of A. thaliana leaf substructures ranging from single-cell trichomes and the interveinal leaf lamina to primary, secondary, and tertiary veins. IR-LAAPPI has also showed its potential for depth profiling analysis by mapping analytes at the different depths of the leaf and spatially resolving the topmost trichomes and cuticular wax layer from the underlying tissues. Traditional MS imaging methods listed in the table above cannot provide this analysis capability.